| 전고체리튬전지용 고전도성의 금속 도핑/치환된 Li7P2S8Br(1-x)Ix 형태의 리튬이온 전도체에 관한 연구 | |||||
| 작성자 | 전** | 작성일 | 2021-11-24 | 조회수 | 262 |
|---|---|---|---|---|---|
|
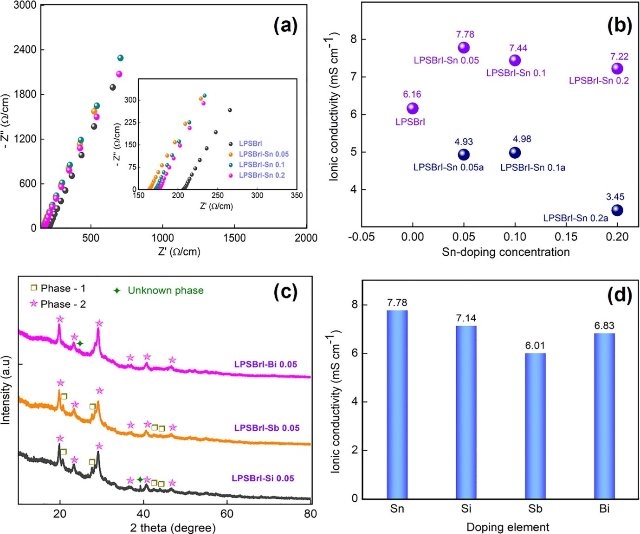
Fig. 6. (a) Nyquist plot of pure LPSBrI and Sn doped LPSBrI solid electrolytes, (b) calculated ionic conductivity values of pure LPSBrI and Sn doped LPSBrI solid electrolytes, (c) XRD patterns of various metal-doped LPSBrI solid electrolytes (200℃) and (d) calculated ionic conductivity values of various metal-doped LPSBrI solid electrolytes. Preparation of highly conductive metal doped/ substituted Li7P2S8Br(1-x)Ix type lithium superionic conductor for all-solid-state lithium battery applications, Chemical Engineering Journal, 428, 132155(1~12) (2022) DOI : 10.1016/j.cej.2021.132155 Abstract Sulfide-based lithium superionic conducting solid electrolytes for all-solid-state lithium batteries have received much attention due to their high safety, ionic conductivity, and compatibility. However, high-temperature treatment over a long time is needed to achieve high ionic conductivity. In this work, we have prepared the dual-halide-based Li7P2S8X type lithium superionic conductors by dry ball mill process and the high ionic conducting phase was achieved by low temperature (200℃) heat-treatment process. High ionic conducting halogen-halogen composition was arrived at through a series of optimization processes. Among the compositions tested, the Li7P2S8Br0.25I0.75 solid electrolyte exhibited a high ionic conductivity value of 6.16 mS cm?1, at room temperature. The ionic radius of the halogen element played an important role in the formation of the high ionic conducting phase. Further, the ionic conductivity of Li7P2S8I0.75Br0.25 solid electrolyte was improved by metal doping at P-site. The Sn-doped Li7P2S8I0.75Br0.25 solid electrolyte exhibited a high ionic conductivity value of 7.78 mS cm?1, at room temperature. The addition of large-sized Sn atoms extended the crystal lattice parameters and increased the Li-ions transport path. The partial substitution of Sn atoms at P-site was confirmed by NMR and Laser Raman analysis. The electrochemical stability of the prepared solid electrolyte was studied by cyclic voltammetry and DC charge-discharge analysis. The addition of a metal atom to the Li7P2S8Br0.25I0.75 solid electrolyte decreased the side reaction with the Li metals. Thus, low-temperature treated metal-doped Li7P2S8Br0.25I0.75 solid electrolytes are highly favourable for the fabrication of high-performance all-solid-state batteries. The fabricated all-solid-state battery using Sn doped Li7P2S8Br0.25I0.75 solid electrolyte exhibited the high specific capacity value of 170 mAh g?1 (0.1C), at room temperature. |
|||||
- 첨부파일
- 2번 게시물.jpg